|
|
|
|
Laman Utama >>Molybdenum karbida
Molybdenum Carbide in Tempered Martensite
Crystal Structure of Mo2C
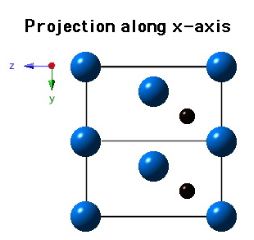
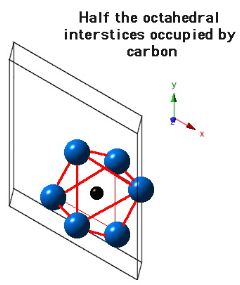
Molybdenum carbide (Mo2C) can occur in many crystalline forms, the most common of which is α-Mo2C and β-Mo2C. It is the latter phase which is stable at low temperatures and occurs in steels. β-Mo2C has a close-packed hexagonal crystal structure with the carbon atoms located in one half of the available octahedral interstices. The lattice parameters assumed for the diagrams below are a=0.3007 nm and c=0.4729 nm.
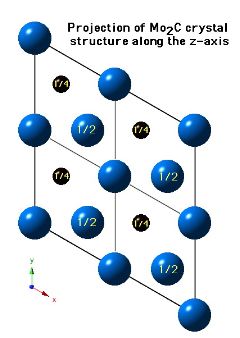
Two molybdenum and one carbon atom per unit cell.
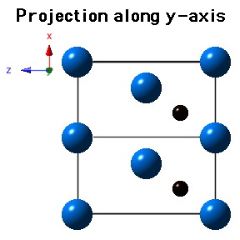
|
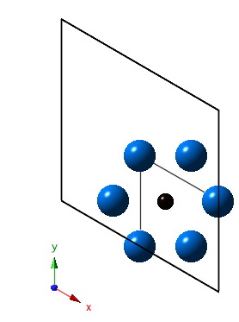
As the projection along the z-axis, but with most of the atoms removed to show carbon in an octahedral interstice.
|
|
In the Mo-C system at least six different phases have been identified, see Table 5.1, this even once called in the literature for "The MoC problem''. The two (possibly three) different MoC phases exhibit an ABAB stacking sequence of the metal planes with carbon in the octahedral sites. The difference between the MoC phases is due to order/disorder transformations of the carbon atoms. The cubic -MoC phase is isostructural with TiC, i.e, a NaCl-type structure, with the well-known ABCABC stacking sequence, while the phase denoted MoC is isostructural with WC and exhibits a simple hexagonal structure with an AAAA packing of the metal atoms. In addition, two more complex phases with hexagonal structures have been identified: the MoC phase with an ABCACB packing sequence and the
-MoC phase with an AABB stacking of the metal planes [10].
Table 5.1: Name, structure and stacking sequence of metallic planes for the six known molybdenum carbide phases
| phase |
structure |
stacking sequence |
| -MoC |
orthorhombic |
ABAB |
| -MoC |
hexagonal |
ABAB |
| -MoC |
hexagonal |
ABCACB |
| -MoC |
cubic |
ABCABC |
| -MoC |
hexagonal |
AAAA |
|
-MoC |
hexagonal |
AABB |
|
The influence of temperature and composition on the stability of different molybdenum carbides is shown in the phase diagram. Several phase diagrams have been published in the literature, but the most recent by Velikanova, Kublii and Khaenko [14] shows that the only thermodynamically stable phases at room temperature are two types of MoC and -MoC (Fig. 5.1). In contrast, -MoC and -MoC are high-temperature phases which become stable at temperatures above about 1700C. The
-MoC phase is considered to be a metastable phase at all compositions and temperatures and is therefore not found in the phase diagram. In fact, the
-MoC has usually been synthesized by carburizing Mo metal with CO and it has been proposed that this phase is stabilized by oxygen and should be considered as an oxycarbide and not a true binary carbide phase [16]. However, recent studies by Lu and coworkers [17] have shown that the
-MoC phase can be deposited as an oxygen-free thin film material and that this phase therefore exists as a true carbide.
Figure 5.1: Phase diagram for the Mo-C system [14].
 |
If you have any interest in molybdenum carbide, please feel free to contact us by email: sales@chinatungsten.com sales@xiamentungsten.com or by telephone:86 592 5129696.
|
|





